Antibiotics
Tenotryl™ (enrofloxacin) injectable solution (Swine)
For treatment and control of SRD and control of colibacillosis
Download the Prescribing Information
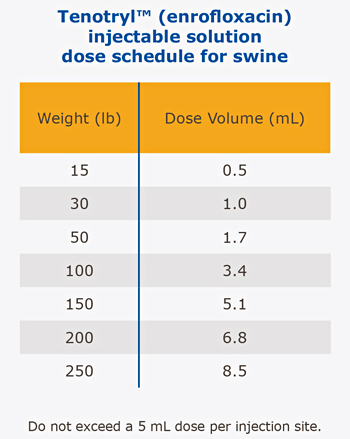
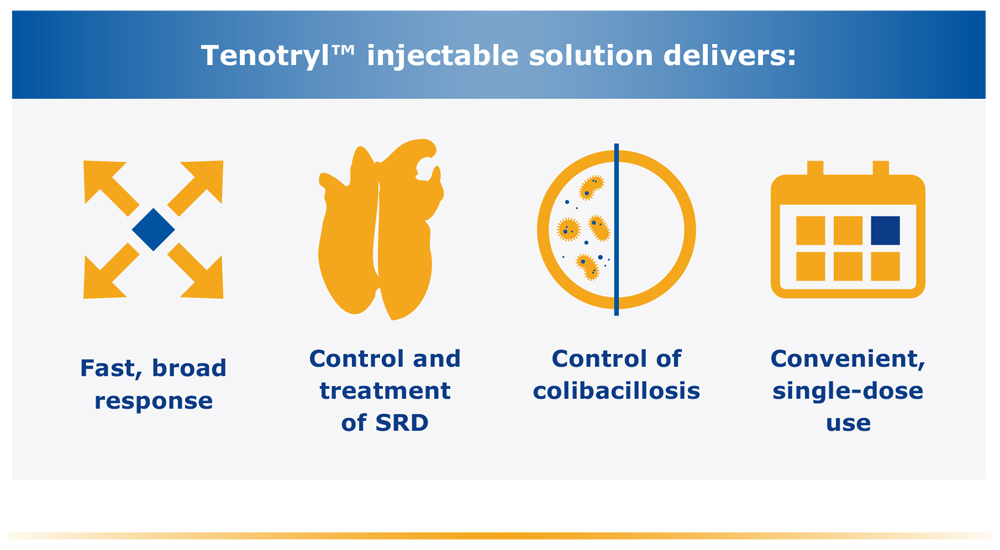
Target multiple bacterial pathogens with a single injection
Reliable, single-dose therapy to provide convenient and comprehensive clinical benefits for key pathogens associated with swine respiratory disease (SRD) and colibocillosis. The effects of enrofloxocin on swine reproductive performance, pregnancy and lactation have not been adequately determined.
Indications:
TenotrylTM (enrofloxacin) injectable solution is indicated for the treatment of swine respiratory disease (SRD) associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis, Streptococcus suis, Bordetella bronchiseptica and Mycoplasma hyopneumoniae. It is also indicated for the control of colibacillosis in groups or pens of weaned pigs where colibacillosis associated with Escherichia coli has been diagnosed. To assure responsible antimicrobial drug use, enrofloxacin should only be used as a second-line drug for colibacillosis in swine following consideration of other therapeutic options.

SWINE IMPORTANT SAFETY INFORMATION
TenotrylTM (enrofloxacin) 100 mg/ml Antimicrobial Injectable Solution:
Not for use in humans. For intramuscular or subcutaneous use in swine. Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian and prohibits the extra-label use of this drug in food producing animals. To assure responsible antimicrobial drug use, enrofloxacin should only be used as a second-line drug for colibacillosis in swine following consideration of other therapeutic options. Animals intended for human consumption must not be slaughtered within 5 days of receiving a single injection dose. The effects of enrofloxacin on swine reproductive performance, pregnancy and lactation have not been adequately determined. The long-term effects on articular joint cartilage have not been determined in pigs above market weight. Subcutaneous or intramuscular injection in swine can cause a transient local tissue reaction and may result in trim loss of edible tissue at slaughter. Quinolone-class drugs should be used with caution in animals with known or suspected Central Nervous System (CNS) disorders.
Tenotryl is labeled for cattle and swine. The information provided on this page is provided specifically
for swine. See the Tenotryl cattle webpage for more information.
Brief Summary of Swine Information
Please see our cattle page.
See additional content for TenotrylTM for Cattle
TENOTRYL is a trademark of Virbac S.A.
References
- Giguére, S.; Prescot, J.F.; Dowling, P.M. 2013. Antimicrobial Therapy in Veterinary Medicine, Fifth Edition.
- Shivdeep SH, et al. 2020 Prevalence and time trend analysis of antimicrobial resistance in respiratory bacterial pathogens collected from diseased pigs in USA between 2006-2016. Research in Veterinary Science. 128:135-144.
- Assunçao P, et al. 2007. Application of flow cytometry for the determination of minimal inhibitory concentration of several antibacterial agents on Mycoplasma hyopneumoniae, J Appl. Microbiol. 102:1132–1137.
- Messenger, K.M. et al. 2011. Distribution of enrofloxacin and its active metabolite, using an in vivo ultrafiltration sampling technique after the injection of enrofloxacin in pigs. J. vet. Pharmacol. Therap. doi: 10.1111/j.1365-2885.2011.01338.x.